A next-generation therapy
Pombiliti® + Opfolda® is the first and only therapy combining a bis-M6P enriched enzyme with an oral enzyme stabilisier1,2
Pombiliti® + Opfolda® has demonstrated efficacy in the first-in-human Phase I/II ATB200-02 and randomised, multicentre Phase III PROPEL (including for as long as 104 weeks in an open-label extension) clinical trials.
How does Pombiliti + Opfolda work?
Different by design for more uptake and complete processing into the most active form of GAA1,3
Pombiliti®Naturally derived* bis-M6P enriched enzyme1,3,4

- bis-M6P has ~3000x the binding affinity to CI-MPR of M6P
- High CI-MPR binding affinity designed for increased uptake into muscle cells
- Can be processed into its most active form to break down glycogen

Opfolda®Oral enzyme stabiliser1-3,5
- Binds with and stabilised Pombiliti® in the blood, which has near-neutral PH that is unfavourable for rhGAA
- Increases the amount of active enzyme that can reach the muscle
The in vitro and pharmacokinetic data supporting these statements cannot be translated into clinical efficacy.
Pombiliti® + Opfolda® was developed to address key challenges in delivering rhGAA with a naturally derived* bis-M6P-enriched enzyme1,5
1. Stability in the blood3,5
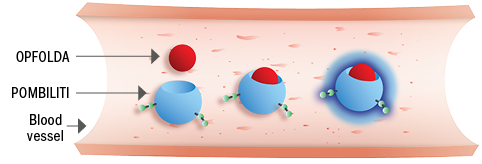
Opfolda® binds with and stabilises Pombiliti® in the blood and increases the amount of active enzyme that can reach the muscle1,3
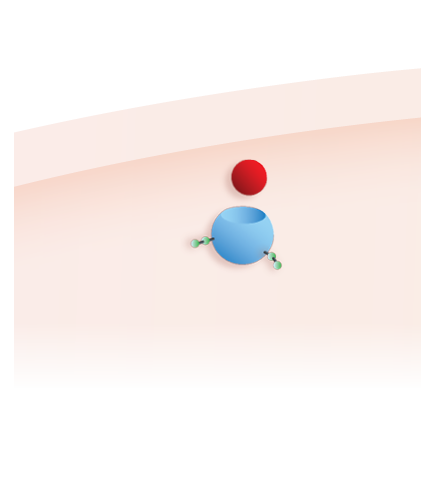
2. Improving uptake3
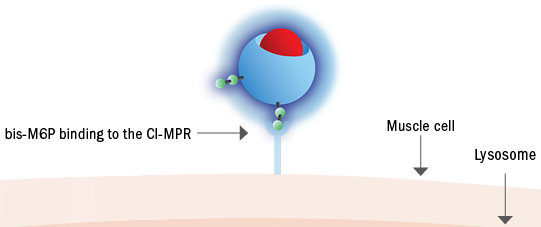
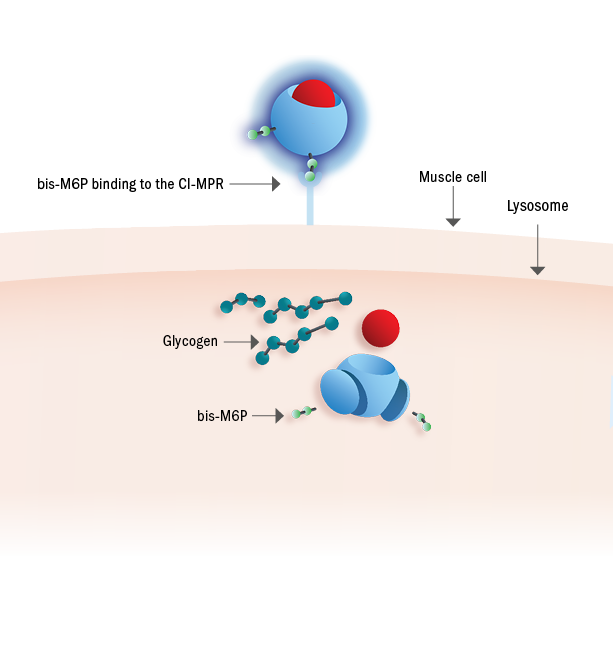
Pombiliti®, a naturally derived* bis-M6P-enriched enzyme, in combination with Opfolda®, binds with high affinity to CI-MPRs on the cell surface to be transported to the lysosome1,2
3. Complete processing in the lysosome1
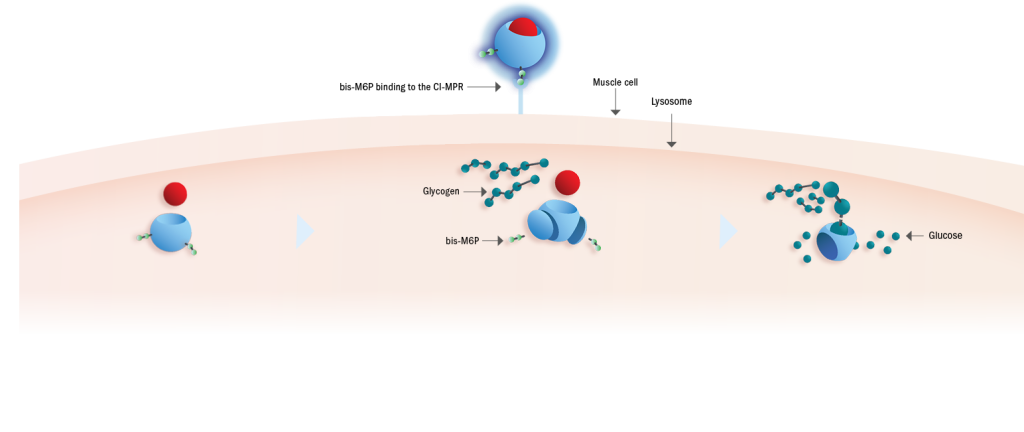
Once inside the lysosome, Opfolda® disassociated from Pombiliti®2

Pombiliti® is completely processed into the most active form of GAA,like endogenous GAA6

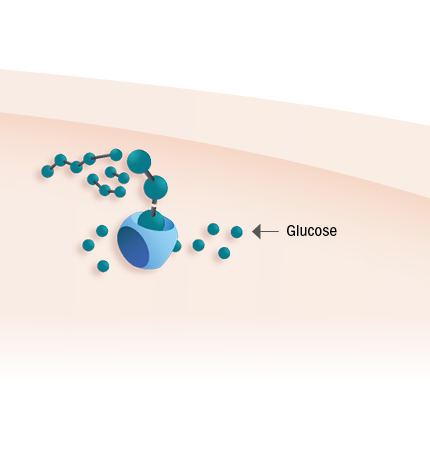
Pombiliti® cleaves glycogen into glucose1
*Enzyme derived from a Chinese hamster ovary (CHO) cell line using perfusion technology, resulting in cellularly (CHO)-derived N-glycans.1

References
- Pombiliti® 105 mg powder for concentrate for solution for infusion. Summary of Product Characteristics.
- Opfolda® 65 mg hard capsules. Summary of Product Characteristics.
- Schoser, B., Roberts, M., Byrne, B.J., et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidasealfa plus placebo in late onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, phase 3 trial. Lancet Neurol. 2021;20(12):1027-1037.
- Do, H.V., Khanna, R. and Gotschall, R. Challenges in treating Pompe disease: an industry perspective. Ann Transl Med. 2019;7(13):291.
- Johnson, F.K., Kang, J., Mondick, J., et al. Mechanism of action, plasma total GAA protein PK profiles and PK/PD relationships differ between cipaglucosidase alfa/miglustat and alglucosidase alfa in patients with late onset Pompe disease. Presented at the: 18th Annual WorldSymposium (Abstract No. 166); 2022.
- Selvan, N., Mehta, N., Venkateswaran, S., et al. Endolysosomal N-glycan processing is critical to attain the most active form of the enzyme acid alpha glucosidase. J Biol Chem. 2021;296:100769.