In a pharmacokinetics study of Pombiliti® in the blood in enzyme-replacement therapy (ERT)-experienced adults with late-onset Pompe disease (LOPD), it was found that 29% more Pombiliti® was available in the blood with the addition of Opfolda® compared to without.1,2
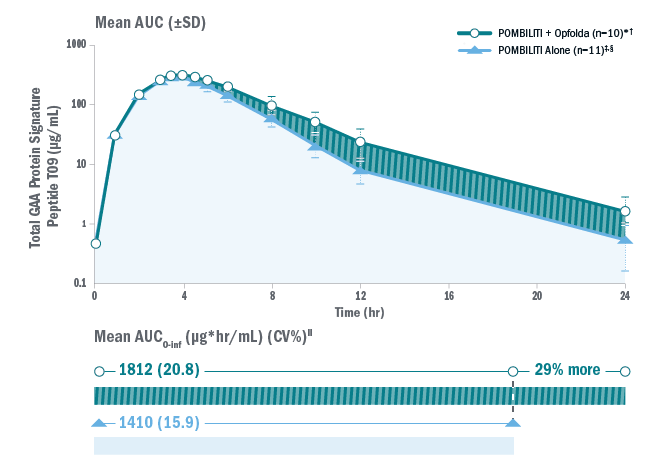
NB, the pharmacokinetic data in this study cannot be directly translated into clinical efficacy.
§Pombiliti® is not approved for use without Opfolda®.
*One subject was not dosed properly with Opfolda® and was excluded from the analysis.2
†Pombiliti® 20 mg/kg + Opfolda® 260 mg.
‡20 mg/kg.
‖AUC0-inf is area under the curve (from time 0 to infinity), representing total drug exposure over time.
AUC: area under the curve; CV: coefficient of variation; ERT: enzyme replacement therapy; GAA: acid alpha-glucosidase; LOPD: late-onset Pompe disease; SD: standard deviation.
An open-label Phase I/II study to assess the safety, efficacy and pharmacological profile of Opfolda® co-administered with Pombiliti® in adult patients with Pompe disease who were naïve to enzyme replacement therapy (ERT) or had received previous ERT for at least two years3,4
ATB200-02 study design
Adults aged 18–75 with a confirmed diagnosis of Pompe disease were eligible for the study4, which was conducted in four stages. Stages 1 and 2 assessed ascending single doses of Pombiliti® and ascending dose combinations of Pombiliti® + Opfolda® in ambulatory patients who had taken prior ERT for 2 – 6 years (n=11).3
Stage 3 is an ongoing long-term (24 month) safety and efficacy study of Pombiliti® 20 mg/kg + Opfolda® 260 mg. A total of 29 patients were enrolled, divided into four cohorts:3
- Cohort 1: Patients from Stages 1 and 2 (n=11)3
- Cohort 2: Non-ambulatory, prior ERT ≥2 years (n=6)3
- Cohort 3: Ambulatory, ERT-naïve (n=6)3
- Cohort 4: Ambulatory, prior ERT ≥7 years (n=6)3
Stage 4 is an open-label extension with patients continuing Pombiliti® + Opfolda® treatment.4
Patients were generally representative of the adult Pompe disease population.3
Please note, no formal sample size calculation was conducted for this study – it was designed as a first-in-human trial without statistical hypothesis testing or comparisons. A sample size between 18 to 34 patients was deemed sufficient.3
The study did not use inferential statistics. Instead, continuous variables were summarised through descriptive statistics. Given the staggered enrolment of patients, the number of participants with available data decreases over time. However, these patients are still participating in the study.3
Long-term data (up to 48 months) for ATB200-02 were reported in 2023.3
Study endpoints
Primary endpoints: 4
- Plasma pharmacokinetics
- Safety & tolerability (treatment-emergent adverse events [TEAEs], infusion-associated reactions [IARs])
Other assessments included:3
- Antibody levels
- Pharmacodynamics (including CK and Hex4 biomarkers)
- Efficacy (including sitting forced vital capacity [FVC], 6-minute walk distance [6MWD] and manual muscle test score [MMT])
- Patient-reported outcomes
- Laboratory markers
Increased Pombiliti® exposure with Opfolda®
Compared with Pombiliti® alone, co-administration of Opfolda® with Pombiliti®:
- resulted in greater area under curves for total acid α-glucosidase (GAA) protein exposure;3,5
- increased the distribution half-life of total GAA by 47%.3
Overall, this indicates stabilisation of Pombiliti® by Opfolda® in the blood.3
Motor function
Improved motor function*
- 6MWD: Both the ERT-experienced and ERT-naïve cohorts showed durable mean improvements from baseline in % predicted 6MWD up to 48 months. At Month 48, mean change from baseline was +5.9% for ERT-experienced and +11.7% for ERT-naïve patients.3 After 12, 24, 36 and 48 months of follow-up, distance walked improved numerically from baseline for 13/16, 9/13, 6/12 and 6/9 of the ERT-experienced patients, and 6/6, 6/6, 4/5 and 4/4 of the ERT-naïve patients, respectively.3
- MMT: In ambulatory patients, mean change in lower extremity MMT score improved numerically from baseline in both ERT-experienced and ERT-naïve cohorts and improvements were maintained for up to 48 months.3 At Month 48, mean change in MMT score from baseline was 3.5 for ERT-experienced patients and 1.0 for ERT-naïve patients.3
*NB: no formal sample size calculation was conducted for this study – it was designed as a first-in-human trial without statistical hypothesis testing or comparisons. A sample size between 18 to 34 patients was deemed sufficient.3
Respiratory function
Stable or improved respiratory function*
- ERT-experienced: FVC mean change from baseline was +1.0% of predicted at Month 48 and had generally remained stable throughout the study.3 After 12, 24, 36 and 48 months of follow-up, mean change in FVC from baseline improved by ≥3% or remained stable (±3%) for 9/16, 11/13, 8/10 and 4/6 patients, respectively.3
- ERT-naïve: Mean change from baseline in FVC at Month 48 was +8.3% of predicted, and improvements from baseline were generally stable throughout the study.3 After 12, 24, 36 and 48 months of follow-up, mean change in FVC from baseline improved or remained stable for 5/6, 6/6, 5/5 and 4/4 patients, respectively.3
*NB: no formal sample size calculation was conducted for this study – it was designed as a first-in-human trial without statistical hypothesis testing or comparisons. A sample size between 18 to 34 patients was deemed sufficient.3
Adverse reactions
- All patients experienced TEAEs.3 The most common included fall, nasopharyngitis, diarrhoea, headache and arthralgia.3 Most TEAEs were mild or moderate in severity.3
- 13 patients experienced IARs, four had serious TEAEs potentially related to treatment and two withdrew from the study because of TEAEs. No TEAEs led to death in either group.3
- In general, Pombiliti® + Opfolda® showed a similar safety profile to alglucosidase alfa.3
ERT: enzyme replacement therapy; TEAE: treatment-emergent adverse event; IAR: infusion-associated reaction; FVC: forced vital capacity; 6MWD: 6-minute walk distance; MMT: manual muscle test; CK: creatine kinase; Hex4: glucotetrasaccharides; GAA: acid α-glucosidase.
If you wish to learn more about this clinical data then please contact your local Amicus representative through the Contact us page.
A randomised, double-blind, multicentre Phase III study of the efficacy and safety of Pombiliti® + Opfolda® compared with alglucosidase alfa + placebo in adults with late-onset Pompe disease (LOPD).1,6
Study design
122 adults with confirmed LOPD were included in the 52-week study.1,6 Patients were either naïve to enzyme replacement therapy (ERT; n=27) or had taken alglucosidase alfa for ≥2 years (n=95).1,6
Patients were randomised 2:1 to treatment with Pombiliti® 20 mg/kg + Opfolda® 260 mg* (n=85) or alglucosidase alfa 20 mg/kg + placebo (n=37).1,6 ERT-experienced patients in the Pombiliti® + Opfolda® group (n=65) had taken ERT for a mean of 7.5 years, compared with 7.1 years in the alglucosidase alfa group (n=30).1,6
Baseline characteristics were representative of the LOPD population and generally similar between treatment groups.1,6
Study endpoints
- Primary endpoint: change from baseline to Week 52 in 6-minute walk distance (6MWD) in the Pombiliti® + Opfolda® group vs alglucosidase alfa + placebo.1,6
- Key secondary endpoints: change from baseline to Week 52 in sitting forced vital capacity (FVC), change from baseline to Week 52 in lower extremities manual muscle test (MMT), change from baseline to Week 26 in 6MWD, change from baseline to Week 52 in total score for PROMIS physical function and fatigue, change from baseline to Week 52 in total gait, stairs, Gower’s manoeuvre, chair score (GSGC).1,6
- Various additional secondary endpoints including change from baseline to Week 52 in serum CK and urinary Hex4.1,6
- Safety and tolerability6
Pre-specified subgroup analyses were performed for primary and key secondary endpoints, biomarkers and treatment-emergent adverse events (TEAEs) according to previous ERT status (ERT-experienced or ERT-naïve).6
Motor function
The primary endpoint (mean change in 6MWD from baseline to Week 52) did not reach significance.
Pombiliti® + Opfolda® demonstrated numerical improvement in 6MWD vs baseline in the overall study population (n=122).1
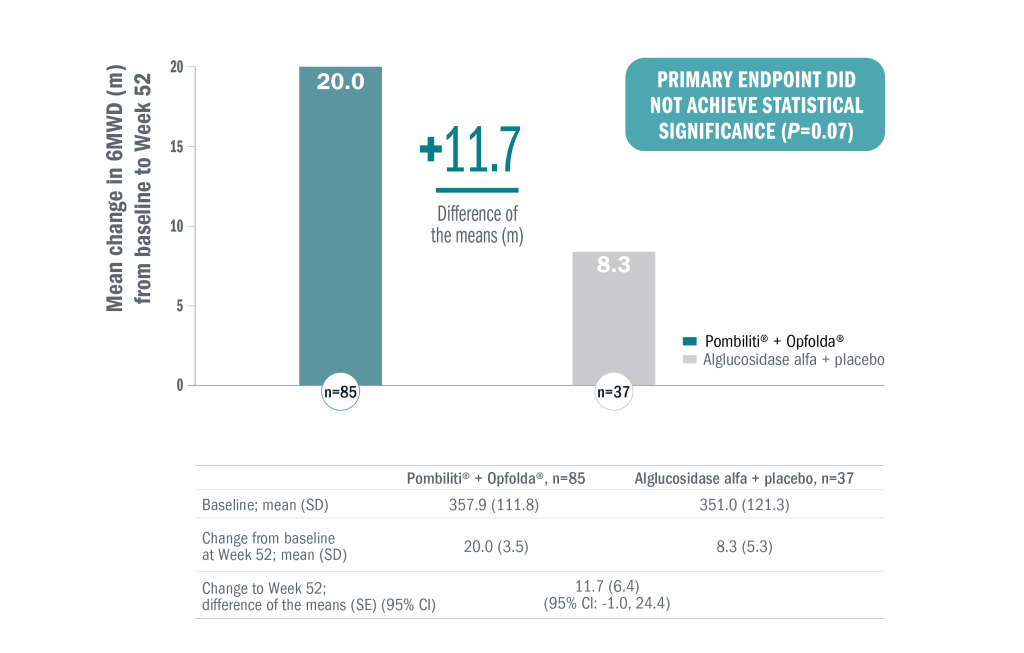
In a post-hoc subgroup analysis:
- ERT-experienced (n=95): numerical improvement in 6MWD from baseline in the Pombiliti® + Opfolda® group compared with alglucosidase alfa + placebo (mean change from baseline to Week 52: 15.9 m and 1.0 m, respectively; mean between-group difference: 14.9 [95% CI: 1.2, 28.6]).1
- ERT-naïve (n=27): numerical improvement in 6MWD from baseline in the alglucosidase alfa + placebo group compared to Pombiliti® + Opfolda®. Pombiliti® + Opfolda® had a mean change from baseline to Week 52 of 28.5 m, compared to 52.7 m for alglucosidase alfa + placebo (mean between-group difference: -24.2 [95% CI, -60.0,11.7]).1,6
Respiratory function
Because the primary endpoint was not significant, all subsequent measures were analysed as nominal statistical assessments of superiority.1
The first key secondary endpoint (change in sitting FVC from baseline to Week 52) demonstrated numerical improvement with Pombiliti® + Opfolda® vs alglucosidase alfa + placebo in the overall study population (n=122).1
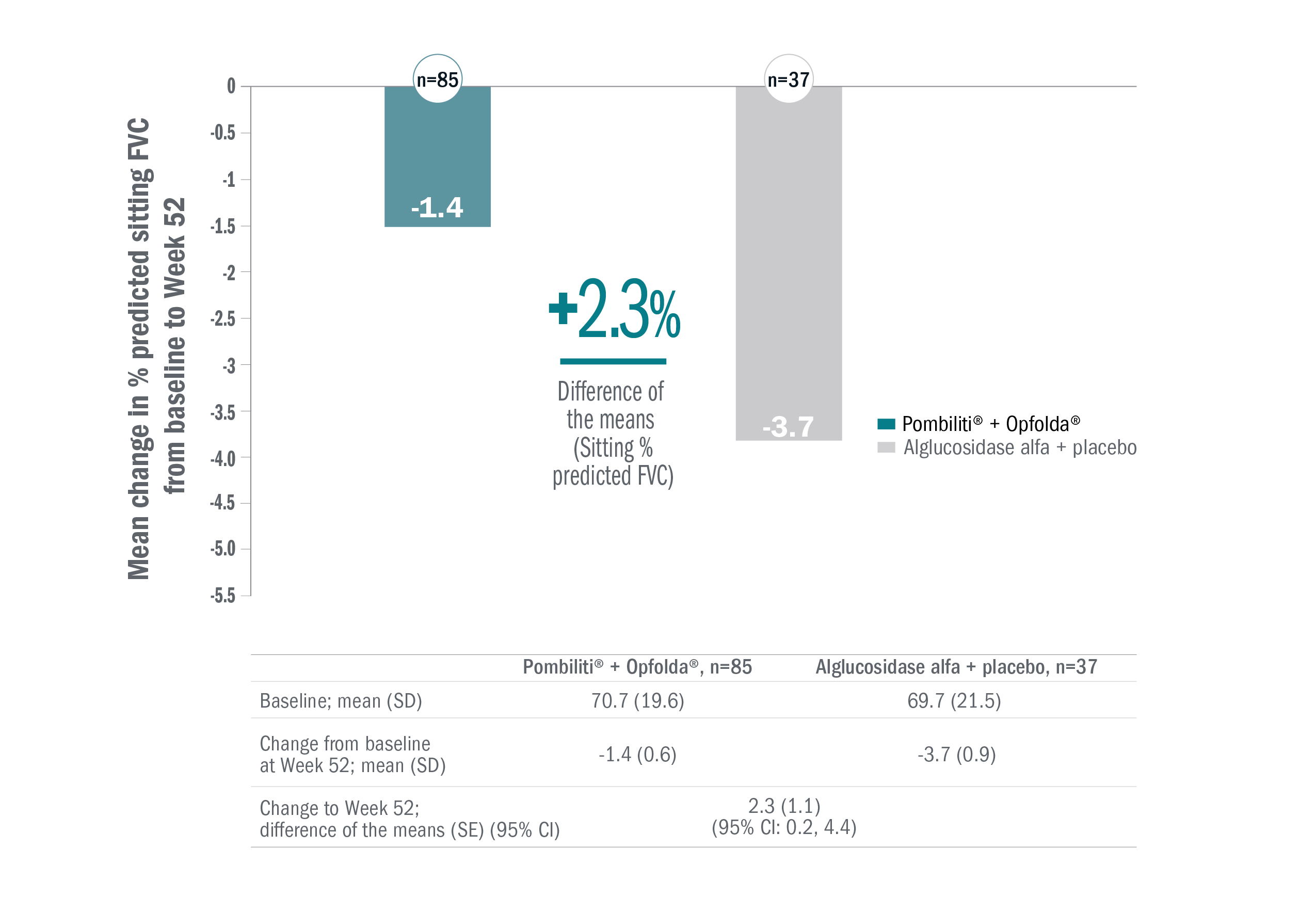
In a post-hoc subgroup analysis:
- ERT-experienced (n=95): FVC stabilised in the Pombiliti® + Opfolda® group and worsened in the alglucosidase alfa + placebo group (mean change from baseline to Week 52: -0.2% vs -3.8%, respectively; mean between-group difference: 3.6 [95% CI: 1.3, 5.9]).1
- ERT-naïve (n=27): FVC declined in both the Pombiliti® + Opfolda® group and alglucosidase alfa + placebo group (mean change from baseline to Week 52: -5.2% vs -2.4%, respectively; mean between-group difference: -2.8 [95% CI: -7.8, 2.3]).1
Other endpoints
A summary of some other key secondary endpoints in the overall study population is included below.6 Because the primary endpoint was not significant, all subsequent measures were analysed as nominal statistical assessments of superiority.6
| Endpoint | Pombiliti® + Opfolda® | Alglucosidase alfa + placebo | Least-square mean difference (95% CI) |
|---|---|---|---|
| Change from baseline at Week 52* Mean (SD,SE); n | Change from baseline at Week 52* Mean (SD,SE); n | ||
| PROMIS physical function score† | 1.9 (7.5, 0.8); 84 | 0.2 (10.8, 1.8); 37 | 1.9 (-1.5 to 5.3) |
| PROMIS fatigue score‡ | -2.0 (5.8, 0.6); 85 | -1.7 (6.6, 1.1); 37 | 0.0 (-2.1 to 2.2) |
| GSGC total score | -0.5 (2.5, 0.3); 72 | 0.8 (1.8, 0.3); 30 | -1.4 (-2.5 to -0.4) |
| Lower MMT score | 1.6 (3.8, 0.4); 80 | 0.9 (2.6, 0.4); 34 | 1.0 (-0.5 to 2.4) |
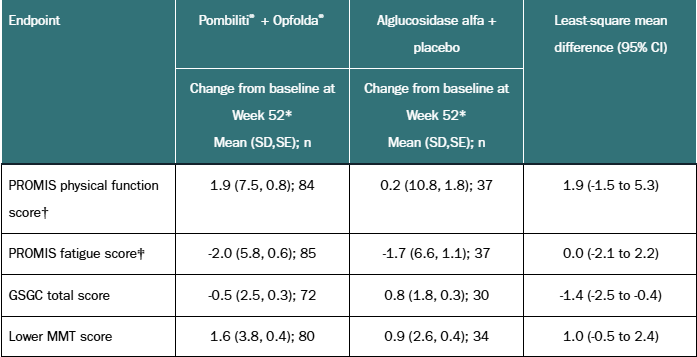
*For lower MMT score, and PROMIS physical function score, a positive change from baseline is favourable. For PROMIS fatigue score and GSGC total score, a negative change from baseline is favourable.
†The PROMIS Physical Function Short Form 20a (v2.0) consists of 20 questions. The first 14 questions are scored on a scale from 1 to 5 with 1=unable to do and 5=without any difficulty. The next 6 questions are scored on a scale from 1 to 5 with 1=cannot do and 5=not at all.
‡The PROMIS Fatigue Short Form 8a includes 8 questions scored from 1 to 5 with 1=not at all and 5=very much; and 2 questions, each scored from 1 to 5 with 1-never and 5=always.
Cl, confidence interval; GSGC, Gait, Stairs, Gower’s manoeuvre, Chair; MMT, manual muscle test; PROMIS, Patient-Reported Outcomes Measurement Information System; SD, standard deviation; SE, standard error.
Greater reductions in serum CK and urinary Hex4 from baseline were seen with Pombiliti® + Opfolda®, compared with alglucosidase alfa + placebo, in the overall population and in the ERT-experienced subgroup:1-7
- Overall population (n=122)
- CK: mean change from baseline to Week 52 was -130.5 vs 60.2, respectively; mean between-group difference was -190.7 (p<0.001).6,7
- Hex4: mean change from baseline to Week 52 was -1.9 vs 1.2, respectively; mean between-group difference was -3.1 (p<0.001).6,7
- ERT-experienced (n=95):
- CK: mean change from baseline to Week 52 was -118.0 vs 79.6, respectively; mean between-group difference was -197.6 (p<0.001).7
- Hex4: mean change from baseline to Week 52 was -1.7 vs 1.9, respectively; mean between-group difference was -3.6 (p<0.001).6,7
The ERT-naïve subgroup (n=27) also showed greater reductions in serum CK and urinary Hex4 with Pombiliti® + Opfolda® than with alglucosidase alfa + placebo (p<0.0001 for both comparisons).6
Adverse reactions
- Pombiliti® + Opfolda® was generally well-tolerated, with a similar overall safety profile to alglucosidase alfa + placebo.6
- 96% of patients (118 of 123) had ≥1 TEAE, and incidence was similar between the groups (95% for Pombiliti® + Opfolda® and 97% for alglucosidase alfa + placebo).6
- The most common TEAEs (affecting ≥15% of patients in either group) were fall, headache, nasopharyngitis, myalgia, arthralgia, nausea and back pain.6
- 25% of patients in the Pombiliti® + Opfolda® and 26% in the alglucosidase alfa + placebo group experienced infusion-associated reactions.6
- One serious TEAE in the Pombiliti® + Opfolda® group (anaphylaxis) was considered potentially related to treatment, and four patients withdrew from the study because of TEAEs (three in the Pombiliti® + Opfolda® group and one in the alglucosidase alfa + placebo group). No deaths from TEAEs were reported.6
*Opfolda® was given at a dose of 260 mg for patients ≥50 kg, 195 mg for patients 40 to <50 kg.1,6
LOPD: late-onset Pompe disease; ERT: enzyme replacement therapy; 6MWD: 6-minute walk distance; FVC: forced vital capacity; MMT: manual muscle test; PROMIS: patient-reported outcomes measurement information system; GSGC: gait, stairs, Gower’s manoeuvre, chair; CK: creatine kinase; Hex4: glucotetrasaccharides; TEAE: treatment-emergent adverse event; SE: standard error; CI: confidence interval
If you wish to learn more about this clinical data then please contact your local Amicus representative through the Contact us page.
The ongoing open-label extension (OLE) part of the Phase III PROPEL trial where patients either continued on, or were switched to, Pombiliti® + Opfolda®.
Study design
82 of 85 patients from the Pombiliti® + Opfolda®* arm continued their treatment into the OLE. Additionally, all 37 patients in the alglucosidase alfa + placebo arm switched to Pombiliti® + Opfolda®*. The OLE ran from PROPEL Week 52 to Week 104.8
As in PROPEL, baseline characteristics in the OLE were representative of the population of people with late-onset Pompe disease (LOPD) and were similar between treatment groups.8
Study endpoints
Key endpoints (reported as change from PROPEL baseline to Week 104):8
- Motor function, measured by 6-minute walk distance (6MWD) (% predicted)
- Respiratory function, measured by sitting forced vital capacity (FVC)
(% predicted) - Biomarkers, hexose tetrasaccharide (Hex4) (mmol/mol) and creatine kinase (CK) (U/L)
- Safety: treatment-emergent adverse events (TEAEs) and infusion-associated reactions (IARs)
This single-arm OLE study had no control group and there were no formal hypotheses or sample size calculations. Descriptive statistics only have been used.
ERT-experienced patients: 6MWD and FVC
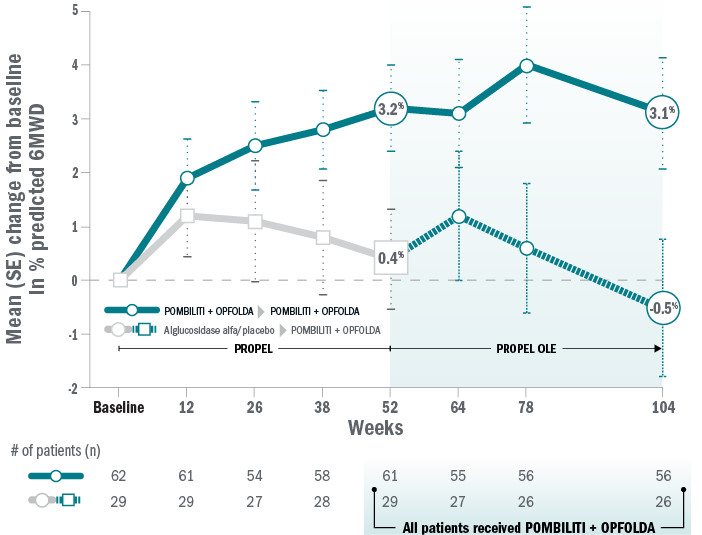
Mean change for ERT-experienced patients on Pombiliti® + Opfolda® in % predicted 6MWD from baseline to Week 104.8
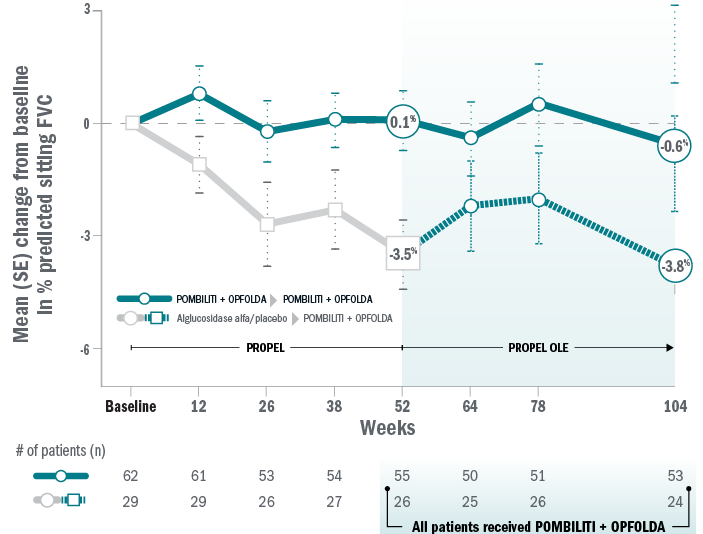
Mean change for ERT-experienced patients on Pombiliti® + Opfolda® in sitting % predicted FVC from baseline to Week 104.8
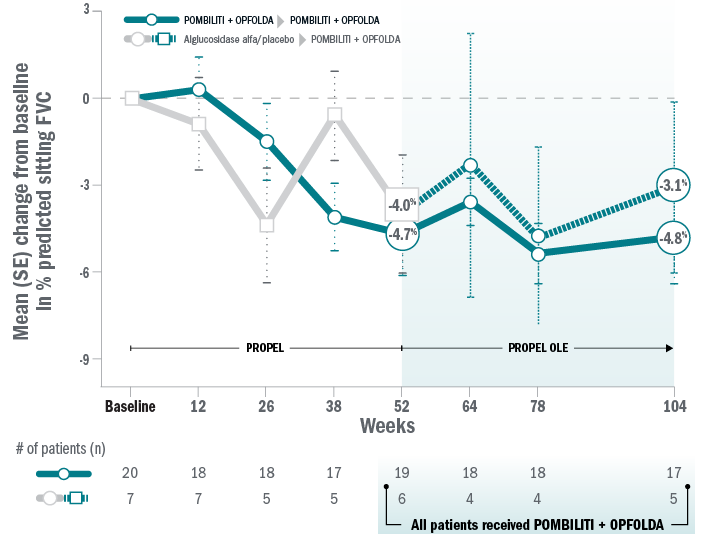
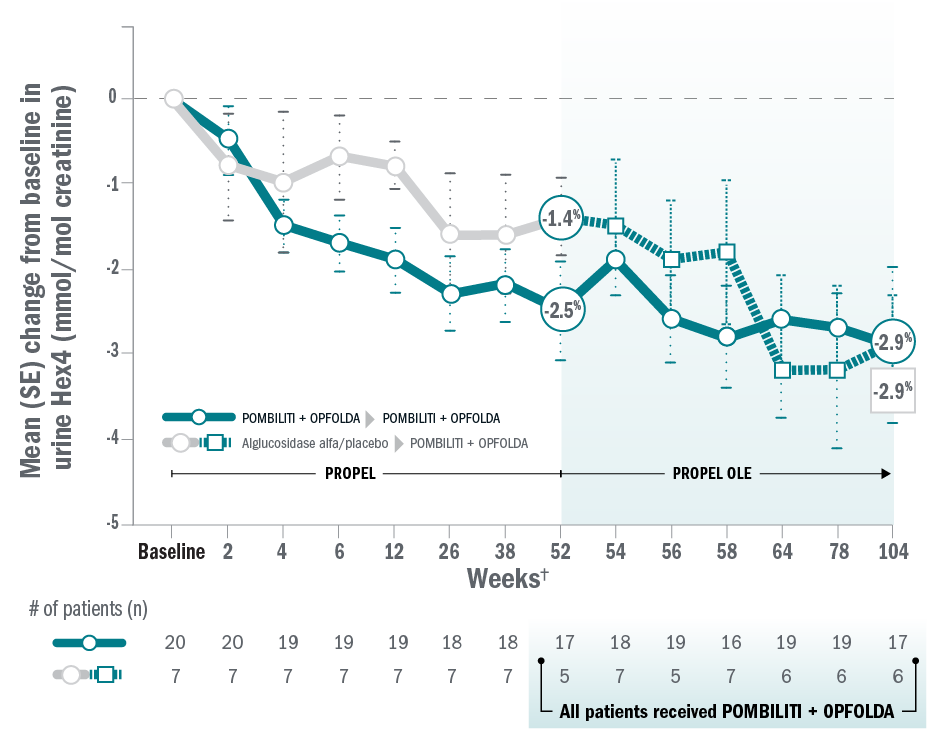
ERT-naïve patients: 6MWD and FVC
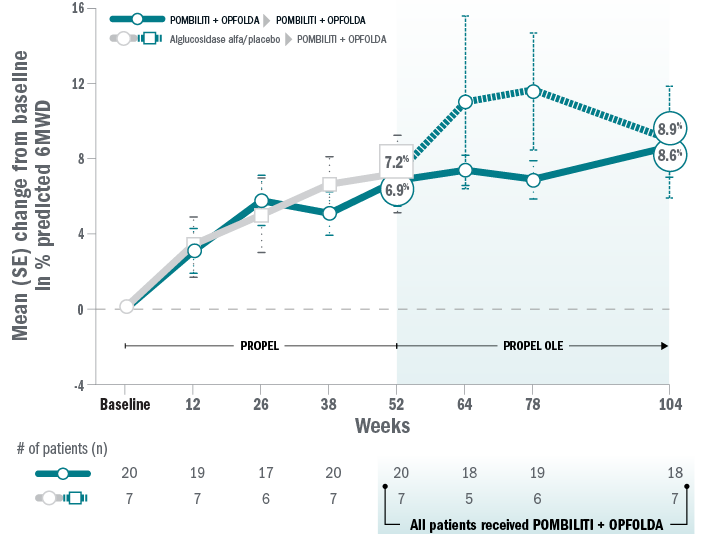
Mean change for patients on Pombiliti® + Opfolda® in percent predicted 6MWD from baseline to Week 104:8
Mean change for patients on Pombiliti® + Opfolda® in sitting percent predicted FVC from baseline to Week 104:8
ERT-experienced patients: biomarkers
Mean change for ERT-experienced patients on Pombiliti® + Opfolda® in Hex4 from baseline to Week 104.8
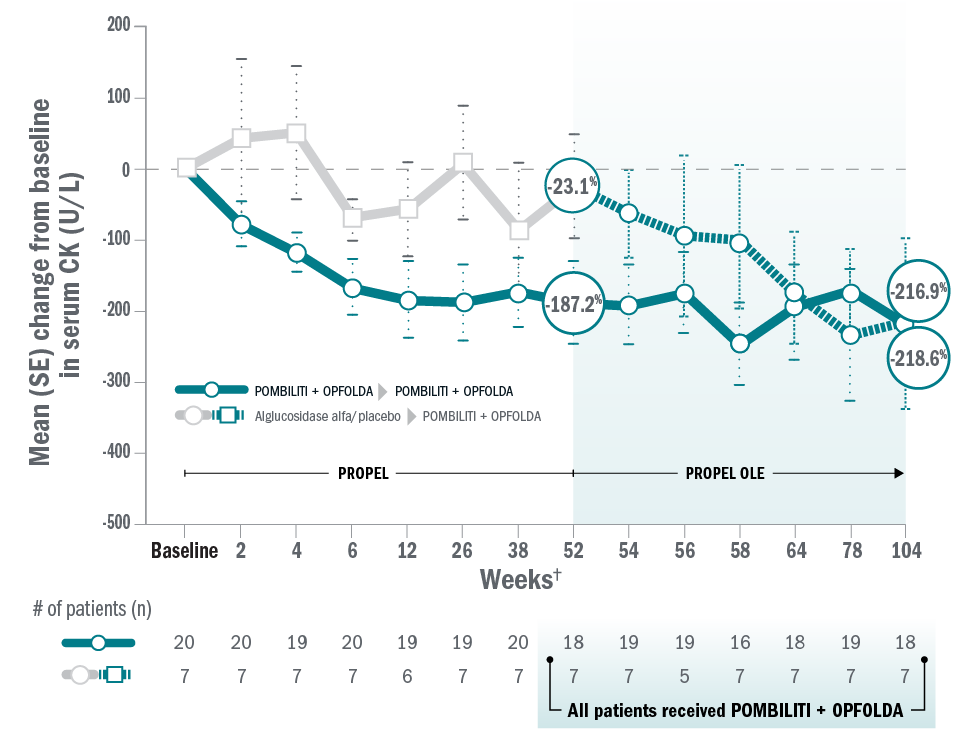
Mean change for ERT-experienced patients on Pombiliti® + Opfolda® in CK from baseline to Week 104.8
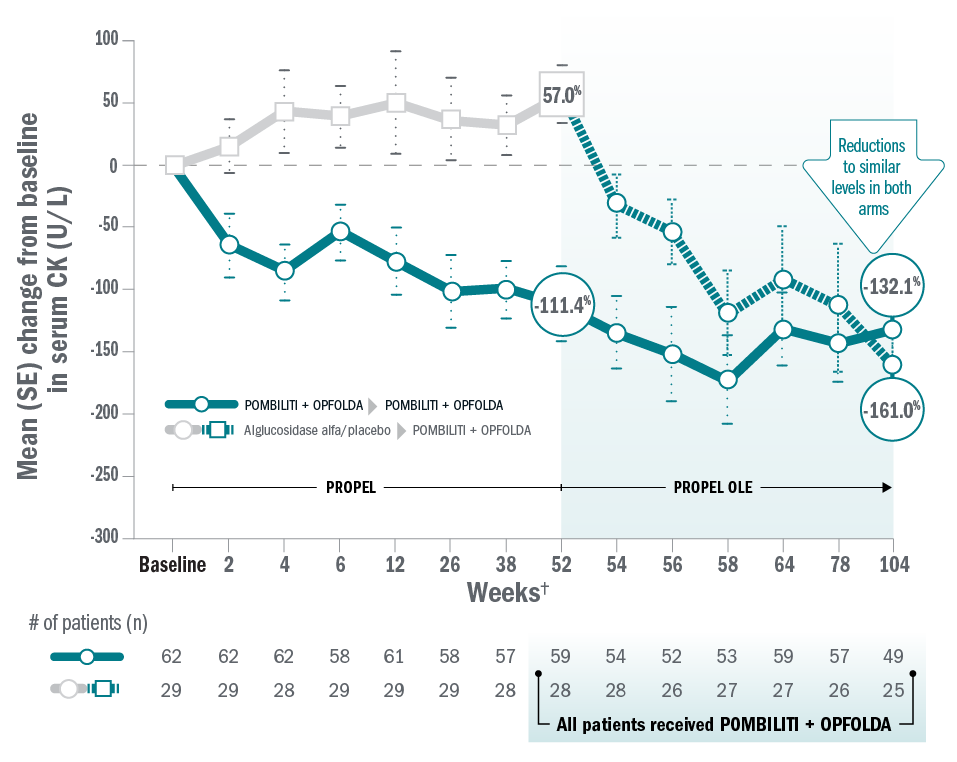
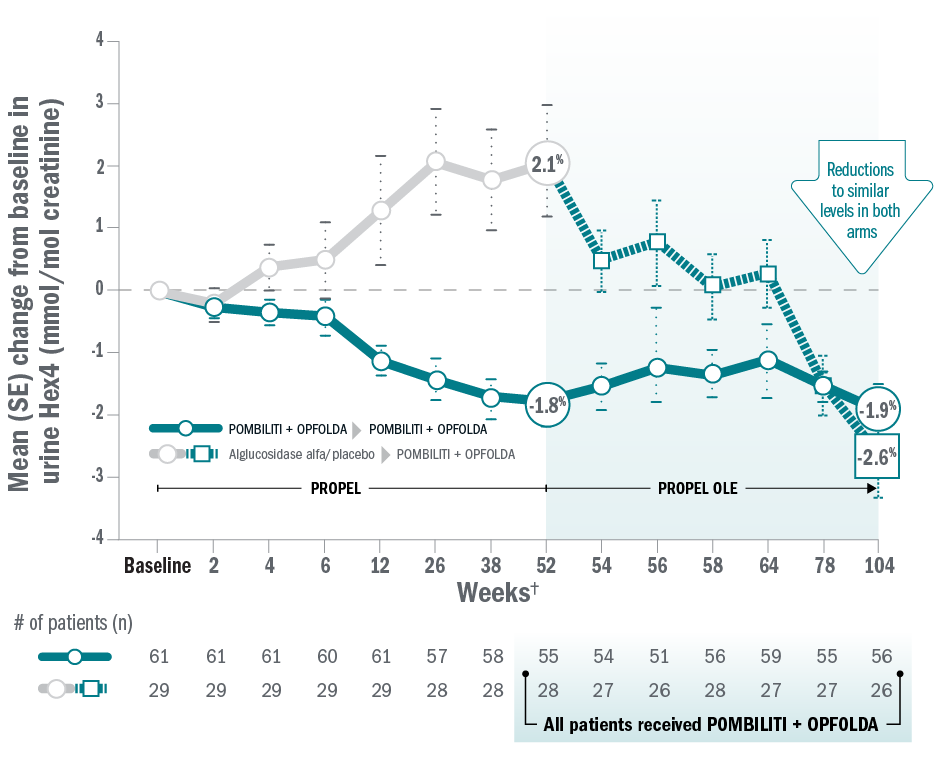
ERT-naïve patients: biomarkers
Mean change for patients on Pombiliti® + Opfolda® in Hex4 from baseline to Week 104:8
Mean change for patients on Pombiliti® + Opfolda® in CK from baseline to Week 104:8
Adverse reactions
- Severe TEAEs – considered to be IARs – included anaphylactoid reaction, urticaria, pruritus, and chills.8
- The most common TEAEs included headache, diarrhoea, pyrexia, fatigue, nausea, dizziness, pruritus, urticaria, somnolence, upper abdominal pain, abdominal distension, and abdominal pain.8
- Most TEAEs were mild or moderate in severity.8
| Adverse reactions8 | Pombiliti® + Opfolda® (n=85)‡ | Switch (n=37)§ | Total patients treated with Pombiliti® + Opfolda® (N=122) |
| TEAEs, n (%) | 84 (98.9) | 36 (97.3) | 120 (98.4) |
| TEAEs potentially related to treatment | 37 (43.5) | 15 (40.5) | 52 (42.6) |
| TEAEs potentially related to treatment leading to study discontinuation | 3 (3.5)‖ | 2 (5.4)¶ | 5 (4.1) |
| Serious TEAEs | 14 (16.5) | 6 (16.2) | 20 (16.4) |
| Serious TEAEs potentially related to treatment | 1 (1.2) | 2 (5.4) | 3 (2.5) |
| TEAEs leading to study withdrawal during OLE | 1 (1.2) | 2 (5.4) | 3 (2.5) |
| IARs | 27 (31.8) | 10 (27.0) | 37 (30.3) |
*Pombiliti® + Opfolda® dosage: 20 mg/kg Pombiliti® IV + 260 mg or 195 mg Opfolda® orally Q2W (260 mg Opfolda® for patients weighing ≥50 kg and 195 mg for patients weighing ≥40 kg to <50 kg.
†Timeline on x axis not to scale.
‡Includes data from PROPEL and the OLE. Patients may or may not have continued Pombiliti® + Opfolda® in the OLE.
§Includes data from the OLE only.
‖Two patients discontinued treatment during PROPEL due to anaphylactoid reaction and chills, respectively, and one patient discontinued treatment during the OLE due to urticaria.8
¶Two patients discontinued from the OLE due to urticaria and hypotension, and anaphylaxis, respectively.8
6MWD: 6-minute walk distance; CK: creatine kinase; ERT: enzyme replacement therapy; FVC: forced vital capacity; Hex4: hexose tetrasaccharide; IAR: infusion-associated reaction; LOPD: late-onset Pompe disease; OLE: open-label extension; TEAE: treatment-emergent adverse event.
If you wish to learn more about this clinical data then please contact your local Amicus representative through the Contact us page.

References
- Pombiliti® 105 mg powder for concentrate for solution for infusion. Summary of Product Characteristics.
- Data on file, Amicus Therapeutics, Inc.
- Byrne BJ, Schoser B, Kishnani PS, et al. Long-term safety and efficacy of cipaglucosidase alfa plus miglustat in individuals living with Pompe disease: an open-label phase I/II study (ATB200-02). J Neurol. 2024;271(4):1787-1801.
- ClinicalTrials.gov. First-in-human study to evaluate safety, tolerability, and PK of intravenous ATB200 alone and when co-administered with oral AT2221. Amicus Therapeutics, ClinicalTrials.gov Identifier: NCT02675465. June 2022. https://clinicaltrials.gov/study/NCT02675465?id=NCT02675465&rank=1. Accessed November 2025.
- Byrne BJ, Schoser B, Kishnani PS, et al. Long-term safety and efficacy of cipaglucosidase alfa plus miglustat in individuals living with Pompe disease: an open-label phase I/II study (ATB200-02). J Neurol. 2024;271(4):1787-1801. [Suppl appendix].
- Schoser B, Roberts M, Byrne BJ, et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, Phase III trial. Lancet Neurol. 2021;20:1027-1037.
- Schoser B, Roberts M, Byrne BJ, et al. Safety and efficacy of cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo in late-onset Pompe disease (PROPEL): an international, randomised, double-blind, parallel-group, Phase III trial. Lancet Neurol. 2021;20:1027-1037. [Suppl appendix].
- Schoser B, Kishnani PS, Bratkovic D, et al. 104-week efficacy and safety of cipaglucosidase alfa plus miglustat in adults with late-onset Pompe disease: a phase III open-label extension study (ATB200-07). J Neurol. 2024;271(5):2810-2823. doi: 10.1007/s00415-024-12236-0.